Researchers from Indiana University and the University of Maryland School of Medicine have introduced a groundbreaking method to predict cell activity in tissues over time. This technique, likened to weather forecasting models, applies computational modeling combined with genomic technologies to anticipate changes in cell behavior, particularly regarding how cells communicate, which could lead to cancer development.
The innovative software employs a plain-language “hypothesis grammar” that serves as a bridge between biological systems and computational models. This approach allows for effective simulations of cell behavior within tissues. The findings from this research were published in the journal Cell, detailing virtual experiments that examine cancer responses to environmental cells and the developmental layering of the brain.
The project represents a collaborative effort among multiple laboratories, merging software development with clinical and bench science. According to Daniel Bergman, PhD, a scientist at the Institute for Genome Sciences (IGS) and co-lead author of the study, the new grammar facilitates communication not only between biology and code but also among scientists from various disciplines. This could eventually lead to the creation of advanced computer programs designed to select optimal treatments for cancer patients through the development of a “digital twin” of each patient.
The authors concluded that their method effectively “bridges biological, clinical, and systems biology research for mathematical modeling at scale.” They emphasized that cells interact to form ecosystems that evolve as dynamic systems. While recent advances in single-cell and spatial multiomics technologies have enabled the quantification of individual cell characteristics, predicting their evolution remains a significant challenge requiring sophisticated mathematical modeling.
As the researchers noted, traditional bioinformatics and machine learning techniques can identify trajectories and phenotypic changes within individual cell types. Yet, these methods fall short in accounting for complex changes over time within multicellular networks. The team stated, “More advanced computational tools are needed to fill the gaps between measurement times and leverage biological knowledge and mechanisms to forecast unseen emergent behaviors in multicellular systems de novo.”
Jeanette Johnson, PhD, a postdoctoral fellow at IGS, highlighted the limitations of standard biomedical research, which often only captures a single snapshot in time. This is particularly problematic for diseases like cancer, where cellular communication plays a crucial role. Johnson remarked that the immune system’s individualized complexity makes it challenging to extrapolate predictions from human cancer data to specific patients.
Leading the grammar development team, Paul Macklin, PhD, Professor of Intelligence Systems Engineering at Indiana University, explained that this new grammar utilizes natural language statements—referred to as cell rules—to formulate mathematical models. This allows scientists to construct digital representations of multicellular biological systems using simple English sentences. The authors stated, “This enables systematic integration of biological knowledge and multiomics data to generate in silico models.”
Bergman and his colleagues combined this grammar with genomic data from real patient samples to investigate breast and pancreatic cancers. In their study of breast cancer, the team modeled a situation where the immune system fails to control tumor cell growth, leading to increased invasion and cancer spread. They also adapted their computational framework to simulate an immunotherapy clinical trial for pancreatic cancer.
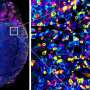
Utilizing genomics data from untreated pancreatic cancer tissue samples, their model revealed that each virtual “patient” exhibited a unique response to immunotherapy. This finding underscores the significance of cellular ecosystems in precision oncology, especially given the challenges posed by pancreatic cancer, which is often enveloped by a dense network of non-cancerous cells known as fibroblasts. The researchers employed new spatial genomics technology to illustrate the communication pathways between fibroblasts and tumor cells, tracking the growth and invasion of pancreatic tumors from actual patient tissues.
The grammar developed by the team is open-source, providing accessibility for the broader scientific community. Bergman emphasized the importance of making this tool available, stating, “By making this tool accessible to the scientific community, we are providing a path forward to standardize such models and make them generally accepted.”
To demonstrate the versatility of this approach, Genevieve Stein-O’Brien, PhD, from Johns Hopkins School of Medicine, applied the grammar in a neuroscience context. The program successfully simulated the creation of layers during brain development.
In summary, the authors noted, “The new conceptual framing (a grammar) for specifying cell behavior hypotheses introduced in this study can systemize and facilitate our thinking of how cells interact to drive tissue ecosystems.” They explored a variety of models related to carcinogenesis, immune responses, tumor growth, and broader implications for neurodevelopment.
Johnson expressed her excitement about the potential of these models, stating that they can be informed by both laboratory and human genomics data. This framework allows for the exploration of hypotheses regarding immune cell behavior over time without incurring additional costs or risks to patients.
Elana J. Fertig, PhD, Director of IGS, remarked on the applicability of principles from weather prediction to biological systems, believing that this approach can yield predictive models for cancer. Fertig described the research as “a tapestry of team science,” with additional validation from clinical collaborators at Johns Hopkins University and Oregon Health Sciences University.
As Mark T. Gladwin, MD, Vice President for Medical Affairs at the University of Maryland, noted, this work paves the way for computerized simulations of bench experiments and clinical trials, allowing for predictions regarding therapy impacts on patients. The implications of this research extend to the development of digital twins and virtual clinical trials, particularly in the field of oncology. Researchers anticipate further advancements in computational modeling of cancer and its applications in clinical settings.