UPDATE: Today marks the 38th anniversary of a groundbreaking scientific discovery that revolutionized chemistry: the identification of buckyballs, the most symmetrical molecules known to science. This milestone occurred on November 14, 1985, at Rice University in Houston, Texas, by chemists Harry Kroto, Richard Smalley, and Robert Curl.
Over a frantic ten-day period, the trio and their graduate students unveiled a new form of carbon, which they later named buckminster fullerene, in honor of inventor Buckminster Fuller. This discovery followed years of research into organic molecules found in interstellar clouds, leading to questions about their origins and compositions.
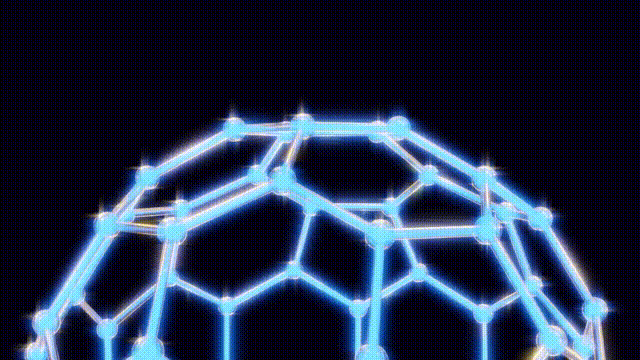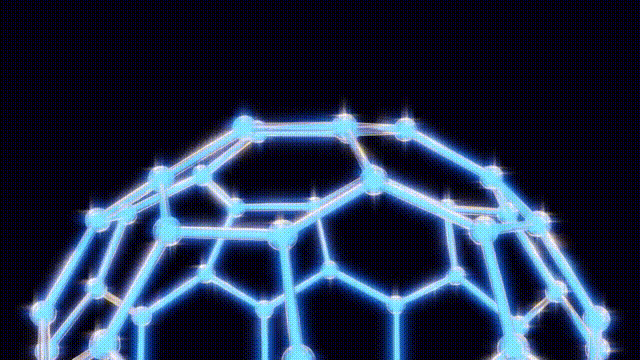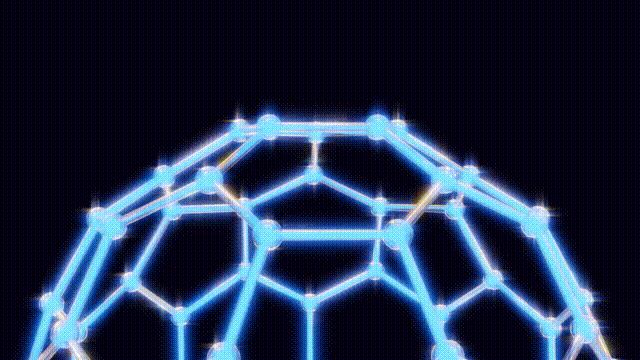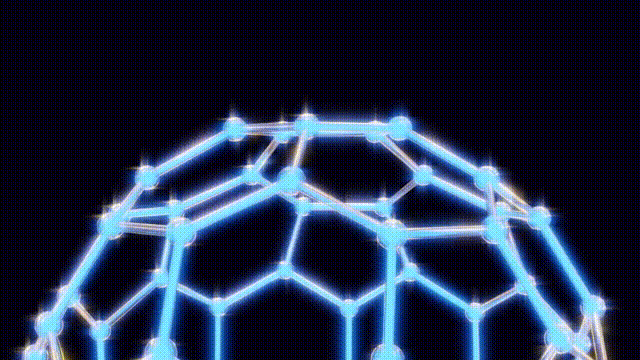
The team’s eureka moment came when Kroto visited Smalley’s lab, where they used a laser to vaporize carbon atoms in a controlled environment. By September 9, 1985, they had produced the unexpected molecule consisting of 60 carbon atoms, which was later playfully dubbed buckyballs.
This discovery has had profound implications for science and technology. The subsequent classification of these molecules, known as fullerenes, has unlocked new avenues in material science, leading to the development of nanotubes that are crucial in various applications, from electronics to medicine. Despite their potential, mainstream applications for buckyballs remain elusive, with ongoing research exploring their role in quantum computing and drug delivery systems.
In recognition of their groundbreaking work, Kroto, Smalley, and Curl were awarded the Nobel Prize in Chemistry in 1996. Their contributions not only advanced scientific understanding but also paved the way for innovations that continue to impact our daily lives.
As we reflect on this milestone, the scientific community remains eager to explore the full potential of buckyballs and their related compounds. The quest for practical applications continues, highlighting the need for ongoing research and collaboration in the field.
Stay tuned for more updates as scientists around the world celebrate this pivotal moment in chemistry and continue to push the boundaries of what is possible with carbon-based materials.