Lithium-metal batteries have gained attention as a leading technology for energy storage due to their ability to deliver significantly more energy in a compact and lightweight form. Despite their promise, a crucial challenge hampers their widespread adoption: the uncontrolled growth of tiny, needle-like structures known as dendrites, which can lead to dangerous short circuits within the battery.
Recent research conducted at the Technical University of Munich (TUM) has revealed unexpected findings about dendrite growth, particularly regarding polymer-based electrolytes. These materials are typically favored for their stability and safety, as they do not leak or ignite. According to Fabian Apfelbeck, a physicist pursuing his doctorate under the guidance of Prof. Peter Müller-Buschbaum, “Electrolytes are responsible for transporting lithium ions back and forth between the two electrodes inside a battery—making the flow of current possible in the first place.”
While polymer-based electrolytes are designed to prevent the formation of dendrites, Apfelbeck’s study, published in Nature Communications, reveals that dendrites can also develop within the polymer electrolyte itself. “Our measurements show that dendrite growth can occur directly inside the polymer electrolyte — right in the material that is actually supposed to protect against dendrites,” he stated.
New Insights into Dendrite Formation
This research challenges the long-held assumption that dendrite growth occurs exclusively at the interface between the electrode and electrolyte. Prof. Müller-Buschbaum expressed surprise at the discovery, noting, “The fact that it also appears far away from that interface surprised us.” This new understanding is pivotal for advancing battery technology, as it provides critical insights into developing materials that can effectively prevent such internal crystallization processes. By addressing this issue, researchers aim to create batteries that are not only safer but also more efficient and longer-lasting.
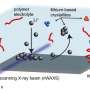
The study utilized advanced techniques to investigate the phenomenon. The team employed nanofocus wide-angle X-ray scattering experiments at the German Electron Synchrotron (DESY) in Hamburg. This method involves using an X-ray beam with a diameter of just 350 nanometers, allowing researchers to visualize microscopic changes within the polymer-based electrolyte during battery operation for the first time. To facilitate this groundbreaking observation, they developed a miniature battery cell that enables real-time monitoring under actual operating conditions.
Implications for Future Battery Development
As the demand for energy storage solutions continues to rise, advancements in lithium-metal battery technology could play a critical role in various applications, from electric vehicles to renewable energy systems. The findings from this research not only highlight a significant barrier to the safe implementation of lithium batteries but also pave the way for innovative approaches to enhance battery performance.
Understanding the mechanisms behind dendrite formation within polymer electrolytes is essential for the future of battery technology. By addressing these challenges, researchers like Apfelbeck and Müller-Buschbaum are working towards safer and more efficient energy storage solutions, which could ultimately transform how we store and utilize energy in our daily lives.