Researchers at La Trobe University have identified a new mechanism through which dying cells can influence immune response and potentially aid in the spread of viral infections. This phenomenon, dubbed the “Footprint of Death,” or FOOD, represents a significant advancement in understanding how cell death can impact both immune function and viral propagation.
When cells undergo apoptosis, a natural form of programmed cell death, they emit signals to attract immune cells, particularly phagocytes, which are responsible for clearing cellular debris. Previous studies have documented various signals, including “find-me” and “eat-me” signals, alongside apoptotic extracellular vesicles (ApoEVs). The recent study, led by Professor Ivan Poon, PhD, head of the laboratory at the La Trobe Institute for Molecular Science (LIMS), reveals a physical trace left behind by dying cells that can enhance immune cell targeting.
According to Professor Poon, “Understanding this basic biological process could open new avenues of research to develop new treatments that harness these steps and help the immune system better fight disease.” The study highlights the crucial role of billions of cells that are programmed to die daily as part of normal bodily processes and disease progression.
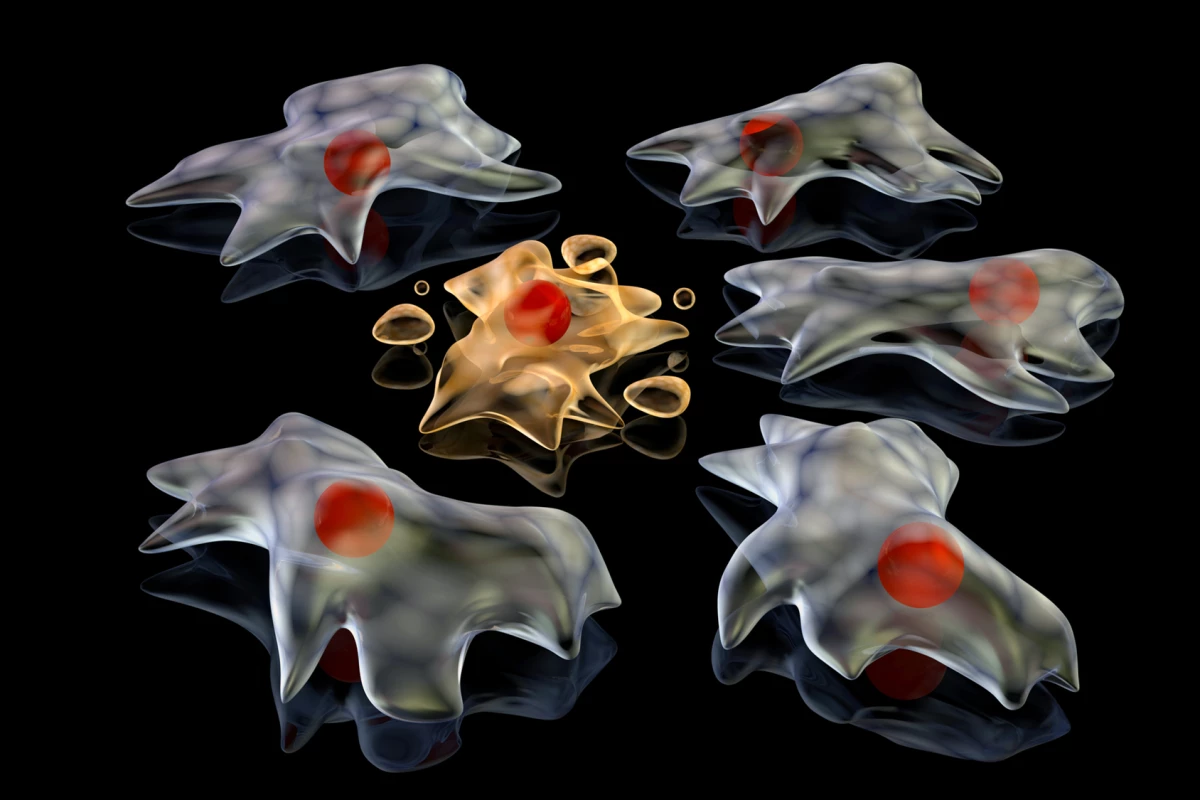
Utilizing a combination of advanced microscopy, live-cell imaging, and proteomics, the researchers explored how FOOD forms and functions. They observed that when adherent cells, those attached to surfaces, die, they retract, leaving behind a thin, membrane-encased footprint. This footprint remains anchored to the surface, unlike other extracellular vesicles that typically disperse.
The study found that FOOD is composed of a high concentration of F-actin, a structural protein, and other adhesion proteins, with minimal nuclear or mitochondrial material present. This suggests that FOOD primarily consists of membrane and cytoskeletal components. When immune cells, specifically macrophages, are exposed to these footprints, they effectively identify and engulf the FOOD-derived extracellular vesicles (F-ApoEVs), facilitating the clearance of dying cells.
Moreover, the research indicates that when cells infected with influenza A undergo apoptosis, both FOOD and F-ApoEVs can contain viral proteins, allowing the virus to potentially use the cell’s death process as a means to spread. This capability suggests that the mechanisms intended for immune response could also be exploited by viruses to enhance their transmission to healthy cells.
Lead author, Stephanie Rutter, a PhD candidate in Poon’s lab, emphasized the unexpected implications of the findings. “We saw F-ApoEVs are readily cleared from the site of cell death. What we didn’t expect was how viruses can also take advantage of this process and cause infection by hiding in F-ApoEVs.”
Despite the groundbreaking insights, the study is not without limitations. Most of the research was conducted in vitro, which raises questions about how FOOD behaves within living organisms. Additionally, the formation of FOOD was primarily observed in adherent cells, leaving its role in non-adherent cells, such as blood cells, uncertain. The long-term fate of FOOD structures within tissues is also yet to be determined, and the findings regarding viral spread are specific to influenza A, with other viruses potentially exhibiting different behaviors.
This research opens the door to new therapeutic strategies that could leverage the role of FOOD in immune response and tissue repair. “The more we understand about cell death and what happens to cells after they die, the better we can understand disease pathologies and find new treatments,” Rutter stated.
The study has been published in the journal Nature Communications, contributing valuable knowledge to the ongoing exploration of cell biology and its implications for health and disease.