Researchers in China have developed a groundbreaking method to convert carbon dioxide (CO2) and methane (CH4) into valuable chemicals using only light, eliminating the need for costly catalysts. This innovative approach harnesses high-energy photons at a wavelength of 185 nm, generated by a specialized 28-W ultraviolet light source, to break the strong chemical bonds present in these greenhouse gases. The findings, published in Nature Photonics on December 14, 2025, present a promising shift in tackling climate change.
Addressing Climate Change with Innovative Solutions
Carbon dioxide and methane account for nearly 84% of the global temperature rise, marking them as leading contributors to climate change. Beyond their role in warming the planet, carbon dioxide is also a primary driver of ocean acidification, jeopardizing marine ecosystems worldwide. To combat these pressing issues, scientists are increasingly seeking methods to not only reduce emissions but also to transform existing greenhouse gases into useful products, fostering a circular economy.
The challenge lies in the chemical resilience of CO2 and CH4, which are notoriously difficult to convert due to their stable molecular structures. Traditional methods often resort to expensive metal catalysts and require extreme conditions, including temperatures exceeding 700 °C and high pressures. Such processes are not only energy-intensive but also costly.
Mechanisms of the Light-Driven Process
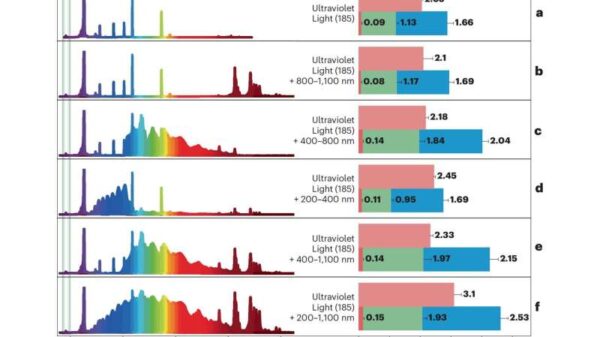
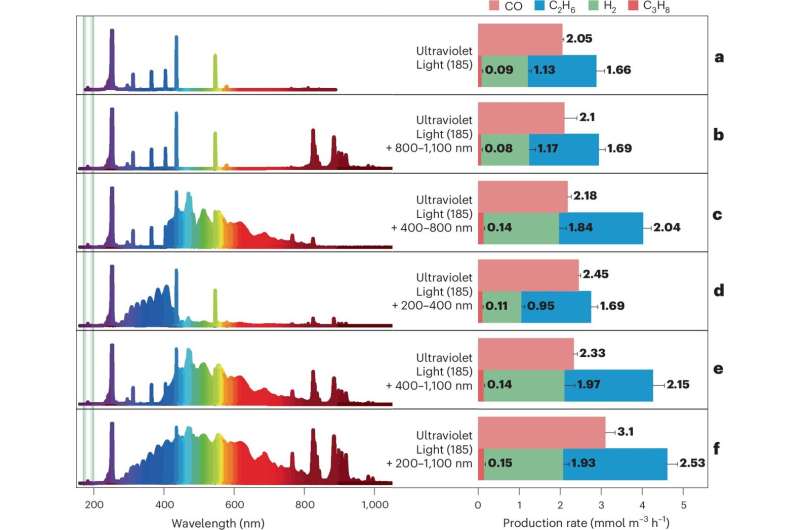
The research team discovered that employing a specific wavelength of light could provide the energy necessary to break the bonds in these gases. They constructed a quartz reactor chamber filled with a mixture of 99.9% pure CO2 and CH4. By illuminating this chamber with various light types under controlled conditions of low pressure and 25 °C, they initiated the conversion process. The high-energy photons at 185 nm, in conjunction with additional light sources ranging from 200–1,100 nm, activated the stubborn gas molecules.
Analysis of gas production revealed the formation of carbon monoxide, hydrogen, and ethane at rates of 3.1 mmol m-3 h-1, 1.93 mmol m-3 h-1, and 2.53 mmol m-3 h-1, respectively. The researchers found that adding water to the gas mixture and removing atmospheric oxygen significantly improved yields. In experiments simulating space-like conditions by flushing the chamber with argon gas, they achieved a total gas conversion of 1.51% within 24 hours.
While the current yield remains low, the researchers express optimism that their findings represent a significant advancement in converting greenhouse gases into valuable products using only light and ambient conditions. This method marks a potential leap forward in addressing climate change sustainably and innovatively.
The research, led by Jianxin Zhai and his team, underscores the importance of developing cost-effective and efficient methods to mitigate greenhouse gas emissions. As the world grapples with climate change, such innovative approaches may offer new pathways to a more sustainable future.