A groundbreaking study from the Institut Pasteur has revealed how aminoglycoside antibiotics penetrate bacterial cells, a discovery that could significantly enhance their effectiveness against resistant strains. Published in Science Advances on September 5, 2025, the research identifies sugar transporters as crucial facilitators of antibiotic entry into bacteria, specifically Escherichia coli, a common pathogen causing severe infections.
The research team, which included scientists from Inserm, CNRS, and Université Paris Cité, demonstrated that aminoglycosides utilize sugar transporters—proteins that typically allow sugars like glucose and fructose to enter bacterial cells. This finding not only elucidates the entry mechanism of these antibiotics but also presents an opportunity to improve their penetration rates, particularly in resistant bacterial strains.
Understanding the Role of Sugar Transporters
Aminoglycosides are known for their effectiveness against a variety of bacteria, including Pseudomonas aeruginosa and Staphylococcus aureus. Their ability to cross the double membrane of gram-negative bacteria like E. coli is vital for disrupting protein synthesis, which leads to bacterial death. Despite their effectiveness, some strains of E. coli have developed resistance, contributing to approximately 829,000 deaths worldwide in 2019.
According to Zeynep Baharoglu, lead author and Research Director at the Institut Pasteur, the mechanism by which aminoglycosides enter bacterial cells has long been debated. Previous theories suggested passive entry through the bacterial envelope. However, the team’s research, which initially focused on stress response in bacteria, opened new avenues for understanding antibiotic transport.
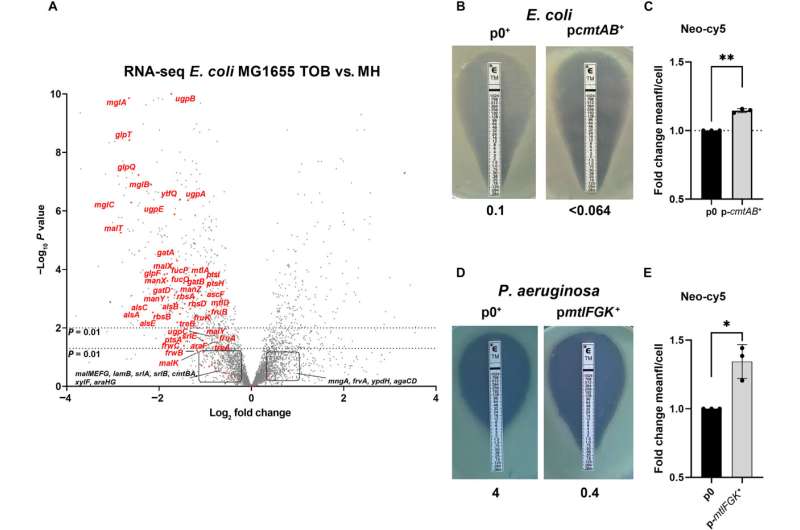
While investigating Vibrio cholerae, the team observed that the antibiotics’ efficacy correlated with the presence of sugar transporters. This prompted further studies on E. coli, leading to unexpected results. “Using fluorescent aminoglycosides, we confirmed they actively enter E. coli through sugar transporters, marking the first evidence of this transport mechanism for antibiotics,” Baharoglu stated.
Enhancing Antibiotic Sensitivity with Uridine
The researchers noted that the abundance of sugar transporters can vary depending on environmental sugar levels. To investigate this further, they tested 200 compounds on human samples contaminated with E. coli and in animal models of urinary tract infections. They identified uridine as a potent candidate that could double the number of sugar transporters in the bacteria.
The results were striking: the introduction of uridine led to a tenfold increase in sensitivity of E. coli to aminoglycosides. The study noted that even drug-resistant strains regained sensitivity to these antibiotics in the presence of uridine. Baharoglu emphasized the significance of this finding, indicating that it could lead to reduced antibiotic dosages, thereby lowering the risk of developing resistance and minimizing side effects associated with high doses, such as kidney and inner ear toxicity.
The implications of this discovery extend beyond E. coli. Similar effects were observed in other bacterial strains, raising hopes for broader applications in treating infections.
Didier Mazel, Head of the Bacterial Genome Plasticity Unit at the Institut Pasteur, highlighted that uridine is already used in clinical practice for various conditions. Its established safety profile in humans allows for quicker clinical trials and potentially reduces the costs associated with bringing new treatments to market.
According to the World Health Organization, antibiotic-resistant bacteria contributed to over 6 million deaths in 2019 alone. The research from the Institut Pasteur underscores the crucial role of fundamental scientific investigation in developing strategies to combat antibiotic resistance effectively.
As clinical trials are anticipated in the near future, this innovative approach could transform how aminoglycosides and potentially other antibiotics are utilized in medical settings, offering hope in the ongoing battle against resistant bacterial infections.