Researchers at the Weizmann Institute of Science have made a significant breakthrough in enzyme design, crafting enzymes that demonstrate catalytic efficiencies exceeding 100,000 M −1 s −1. This achievement allows these novel enzymes to perform with efficiency comparable to that of naturally occurring biocatalysts, without requiring extensive optimization. The findings were published on July 14, 2025, in the journal Nature.
Natural enzymes are known for their ability to facilitate biochemical transformations with remarkable speed and accuracy. However, until now, artificial catalysts have struggled to replicate this level of performance. Through advanced computational strategies, scientists have created proteins capable of catalyzing reactions that are not typically executed by natural enzymes, although these efforts often resulted in much lower conversion rates compared to their biological counterparts.
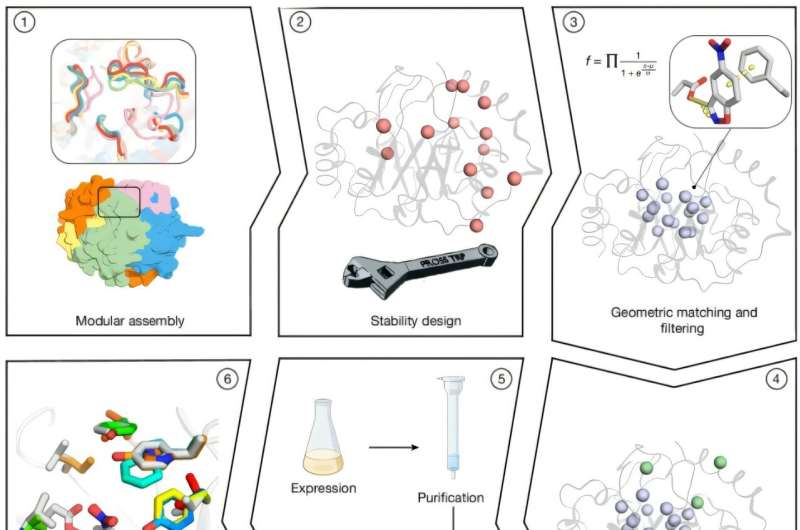
In the study titled “Complete computational design of high-efficiency Kemp elimination enzymes,” the researchers employed a fully computational approach to engineer stable, active enzymes with minimal refinement. They generated thousands of TIM-barrel backbones, a prevalent protein structure among enzymes, by combining fragments from homologous proteins through a process known as combinatorial assembly and design.
The unique structure of the TIM-barrel allows for optimal placement of catalytic and substrate-binding groups. Researchers optimized the active-site residues using Rosetta atomistic calculations, resulting in millions of enzyme designs. These were filtered based on an objective function that balanced energy and desolvation of the catalytic base. Ultimately, 73 designs were selected for experimental testing, with 66 being successfully expressed in soluble form and 14 demonstrating cooperative thermal denaturation behavior.
To enhance catalytic efficiencies, researchers introduced between 5 to 8 active-site mutations per variant. Notably, one variant derived from Des61 achieved a catalytic efficiency (kcat/KM) of 3,600 M −1 s −1 and a turnover rate of 0.85 s −1. Additionally, eight designs based on Des27 surpassed the original catalytic rates by 10–70-fold, with Des27.7 reaching a kcat/KM of 12,700 M −1 s −1 and a turnover rate of 2.85 s −1.
A pivotal single-point mutation that replaced phenylalanine with leucine in the active site resulted in a remarkable increase in catalytic efficiency to 123,000 M −1 s −1 and a turnover rate of 30 s −1. Researchers concluded that the combination of active-site preorganization and the ability to adopt multiple catalytically competent substrate-bound conformations sets this new design apart from prior attempts.
The most successful variant demonstrated exceptional stability, maintaining functionality at temperatures exceeding 85 °C, and achieving catalytic efficiency on par with natural enzymes. The results indicate that current atomistic computational methods are sufficiently reliable to generate efficient enzymes in natural folds, eliminating the need for iterative laboratory screening or dependence on artificial intelligence-generated scaffolds.
Looking ahead, advancements in modeling theoretical enzymes may pave the way for fully programmable biocatalysis. This could open doors to a diverse range of applications, including product manufacturing, therapeutic solutions, pollution remediation, and innovations in green chemistry and large-scale molecular synthesis. The research emphasizes the potential of computational design in revolutionizing enzyme technology, with far-reaching implications for various industries.
This article was authored by Justin Jackson, edited by Sadie Harley, and reviewed by Andrew Zinin, ensuring a high standard of scientific accuracy and integrity. For more detailed insights, the research can be accessed in the publication Nature, alongside further studies on enzyme efficiency.