Research led by Meizhen Wang from Zhejiang Gongshang University has revealed that natural plant extracts can effectively mitigate the risks posed by human bacterial pathogens in agricultural soils. Instead of employing direct bactericidal methods, the extracts interfere with bacterial communication, specifically targeting the quorum sensing (QS) system that allows pathogens to coordinate harmful activities.
The use of manure in agriculture is crucial for maintaining soil fertility and crop productivity. However, it often introduces human bacterial pathogens (HBPs), which can harbor antibiotic resistance genes (ARGs) and virulence factor genes (VFGs). These pathogens can transfer through mobile genetic elements (MGEs) and pose serious risks to both ecosystems and human health. Traditional strategies to manage these risks, such as biochar and engineered nanoparticles, can be costly and may raise environmental concerns.
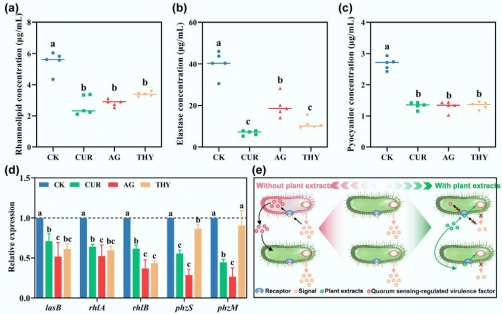
A study published on November 26, 2025, in the journal Biocontaminant highlights a potential solution through the use of plant extracts. The research systematically examined the effects of three plant-derived compounds—curcumin, andrographolide, and thymol—on HBPs in manure-amended soil microcosms. The study utilized metagenomic profiling, targeted gene quantification, and various assays to assess how these compounds influenced the abundance and behavior of HBPs.
Among the findings, the research identified a total of 323 HBPs from a curated pathogen database. The treatment with plant extracts resulted in a reduction of total HBP abundance by approximately 25–28%, specifically targeting pathogens associated with Actinobacteria and Proteobacteria. The overall richness of these pathogens also declined, while alpha diversity remained largely unchanged.
In terms of pathogenicity, the study reported significant reductions in key risk indicators. ARGs decreased by about 20–27%, VFGs by 6–11%, and MGEs by 25–34%. Notably, network analysis showed that high-risk HBPs co-hosting ARGs and VFGs experienced pronounced declines, indicating a potential reduction in their ability to transmit harmful traits.
The research delved into the mechanisms at play, revealing that plant extracts disrupted QS by lowering the abundance of QS-related genes and concentrations of acyl-homoserine lactone signals. This disruption led to a downregulation of QS-regulated genes, translating into decreased virulence factor secretion, up to 40% inhibition of biofilm formation, and as much as 90% suppression of conjugative transfers of ARGs and VFGs.
Molecular docking analyses confirmed that the plant compounds bind to the QS receptor LasR with greater affinity than natural signaling molecules, effectively blocking communication among bacterial populations. This mechanism suggests that the plant extracts act primarily by disarming pathogens rather than killing them, which may help reduce the selective pressure for developing antibiotic resistance.
The implications of these findings are significant for sustainable agriculture. By using plant extracts as environmentally friendly soil amendments, farmers can address the microbial health risks associated with manure application without relying on conventional antibiotics or nanomaterials. This strategy not only contributes to safer food production but also promotes a healthier ecosystem.
The study received funding from multiple sources, including the ‘Leading Goose’ R&D Program of Zhejiang and the National Key R&D Program of China, reinforcing the importance of governmental support in advancing research in agricultural sustainability.
As the search for innovative solutions to combat antibiotic resistance continues, this research paves the way for more sustainable agricultural practices that prioritize both soil health and food safety.