Research has uncovered a pivotal mechanism in the formation of biofilms by the bacterium Pseudomonas aeruginosa, a significant threat to human health due to its antibiotic resistance. This study, published in Nature Microbiology in March 2024, reveals how Pseudomonas utilizes type IV pili to sense sugar trails left by exopolysaccharides (EPS), thereby influencing its motility and biofilm development.
Extracellular polysaccharides play a vital role in biofilm integrity and surface attachment. Among these, the Psl polysaccharide is crucial for Pseudomonas aeruginosa biofilm formation. Previous understanding indicated that during initial biofilm stages, Pseudomonas aeruginosa could detect EPS trails deposited by preceding bacterial cells. This sensing process, facilitated by cyclic diGMP second messengers, guided the bacteria’s movement, although the specific receptors involved remained unidentified.
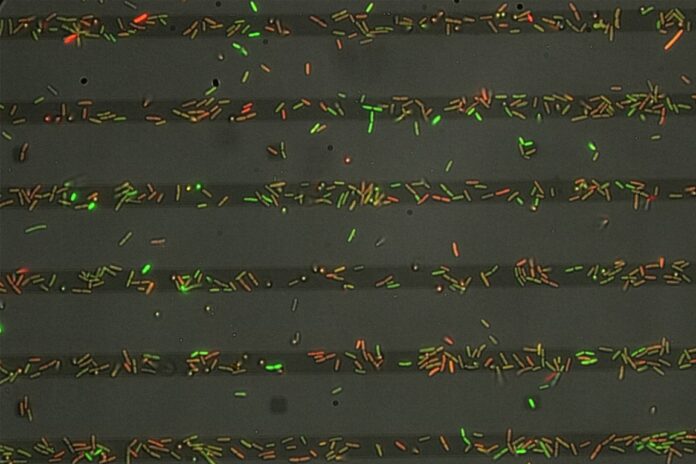
Recent findings demonstrate that Pseudomonas employs type IV pili and adhesins to directly sense these EPS trails. Researchers conducted experiments using bacteria-secreted Psl trails and glycopolymer-patterned surfaces, alongside advanced techniques such as longitudinal cell tracking and genetic mutations. This comprehensive approach revealed that type IV pili enhance the bacteria’s ability to sense EPS through dynamic interactions between pili-powered twitching and specific adhesin–EPS bonds.
According to Gerard Wong, PhD, a professor at the UCLA Samueli School of Engineering, this discovery redefines the role of pili, previously considered primarily as appendages for locomotion. “They also act as sensors that translate force into chemical signals within bacteria, enabling them to identify sugars,” Wong stated. This novel form of signal generation could have significant implications for understanding bacterial behavior.
The implications of this research extend beyond basic science. The findings may lead to new strategies for combating Pseudomonas aeruginosa infections, particularly in patients with cystic fibrosis. “There’s the possibility of turning back the clock on biofilm formation,” said Calvin Lee, PhD, a postdoctoral researcher at UCLA. He suggests that even existing biofilms might be dismantled by encouraging bacteria to self-disassemble.
Beyond healthcare, the study addresses broader challenges posed by biofilms in industrial settings. Biofilms can contaminate pipes, filters, and reactors, as well as contribute to marine biofouling on ships. Wong posed an intriguing question: “Is it possible to make a surface invisible to bacteria?” He speculated that if surfaces could be engineered to mimic empty space, it might be possible to address the significant economic burden posed by biofouling.
Ongoing research aims to identify the range of sugars detected by surface proteins in Pseudomonas and explore how variations in surface shapes influence bacterial movement. The researchers also plan to investigate how these signaling mechanisms persist across generations of bacteria within biofilms.
As William Schmidt, a doctoral student at UCLA, remarked, “We can envision building on these results to influence the bacteria’s behavior.” This could lead to developing strains of Pseudomonas aeruginosa that are more susceptible to antibiotics, potentially easing treatment challenges.
The study not only sheds light on the intricate mechanisms of biofilm formation but also opens avenues for innovative solutions to persistent bacterial challenges in both medical and industrial contexts.