On November 14, 1985, a team of chemists at Rice University made a groundbreaking discovery that would change the landscape of molecular science. They unveiled a new molecule characterized by perfect symmetry, later named buckminsterfullerene, or “buckyballs” for short. This achievement resulted from a decade-long exploration into the nature of carbon molecules and their potential.
The journey began in the late 1970s with Harry Kroto, a lab chemist at the University of Sussex in the United Kingdom. Kroto was investigating the presence of complex organic molecules in the interstellar medium, specifically within vast dark clouds in space. His research revealed an unexpected abundance of carbon chains, contradicting existing astrophysical theories. This anomaly led scientists to consider the possibility that cooling red giant stars were contributing to these unusual carbon formations.
A pivotal moment occurred when Kroto visited the laboratories of chemists Robert Curl and Richard Smalley at Rice University. Smalley had developed a unique apparatus that used a laser to vaporize carbon atoms, allowing them to cool and be analyzed. Inspired by their work, Kroto proposed that the researchers could simulate the conditions of red giants by using a graphite disk instead of metal.
During a remarkable ten-day period in September 1985, the team, which also included graduate students Sean O’Brien and Jim Heath, successfully synthesized six-to-eight carbon chain molecules. While analyzing their results, they stumbled upon unexpected formations of carbon, specifically a molecule comprised of 60 carbon atoms and another containing 70 carbon atoms. These molecules, which Kroto referred to as “uninvited guests,” had been previously identified but largely overlooked by the scientific community.
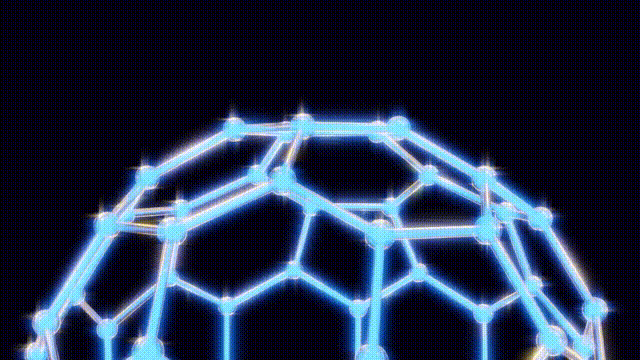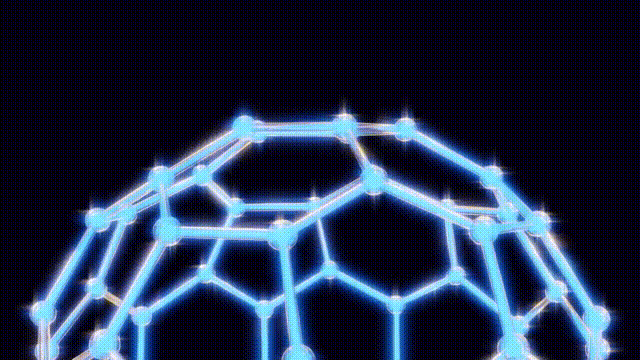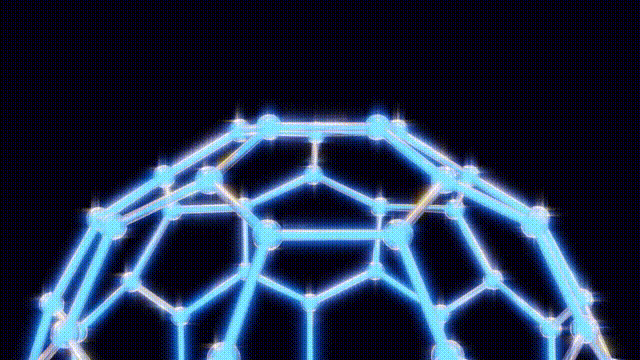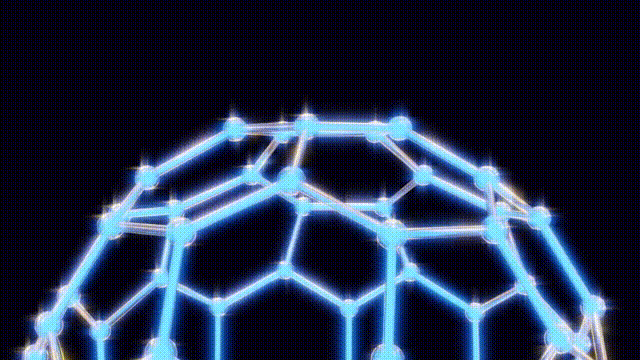
After extensive modeling using everyday materials like toothpicks and jellybeans, the researchers deduced the structure of the C60 molecule. Kroto recalled the geodesic dome designed by futurist Buckminster Fuller, which inspired the naming of their discovery. The final structure was a spherical arrangement of carbon atoms that exhibited remarkable stability and symmetry.
The research team published their findings in the journal Nature, marking a significant milestone in chemistry. Their work laid the groundwork for further exploration of fullerenes, a class of closed carbon molecules. By 1990, scientists had developed techniques to produce buckyballs in larger quantities using electric arcs between carbon rods.
In recognition of their pioneering work, Kroto, Smalley, and Curl were awarded the Nobel Prize in Chemistry in 1996. Their discovery has had far-reaching implications, as fullerenes and their derivatives, such as carbon nanotubes, showcase exceptional strength and conductivity. These materials have found applications in various fields, including atomic force microscopy, energy storage, and biomedical sensing.
Despite the promising potential of buckyballs in areas like quantum computing and drug delivery, mainstream applications remain limited. Researchers continue to explore the possibilities offered by these unique molecules, with the hope that their full capabilities will eventually be realized. The discovery of buckyballs not only expanded the understanding of carbon chemistry but also opened new avenues for scientific inquiry and technological innovation.