Recent research has unveiled a significant mechanism through which cancer cells can influence surrounding healthy cells. Tumors utilize mitochondria, the energy-producing organelles of cells, to reprogram neighboring cells, effectively turning them into allies that support tumor growth. This groundbreaking study, published on August 28, 2023, in the journal Nature Cancer, offers new insights into the interactions between cancer and its environment.
A team led by Sabine Werner, a biochemist and cell biologist at ETH Zurich, discovered that cancer cells can not only steal mitochondria from adjacent cells but also transfer their own mitochondria to nearby healthy cells. This two-way mitochondrial transfer reshapes the behavior and function of these neighboring cells, potentially leading to enhanced tumor growth.
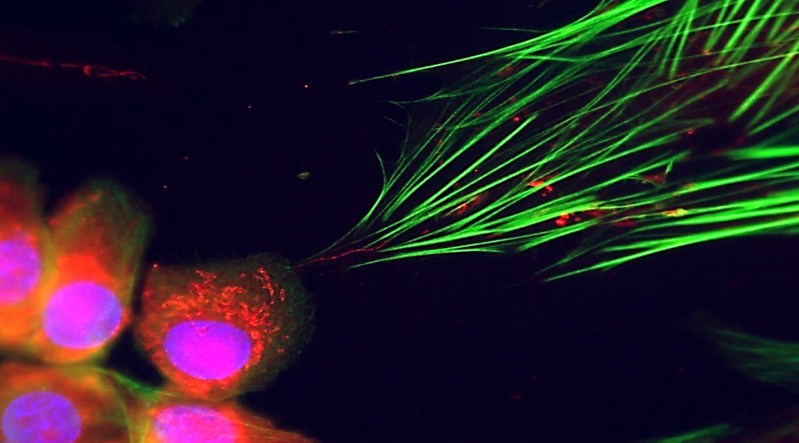
The researchers initially aimed to explore how cancer cells communicate with fibroblasts, a type of connective tissue cell. During their investigation, they uncovered long, slender structures resembling tunneling nanotubes, which serve as conduits for mitochondrial and other cellular exchanges. This phenomenon had not been documented prior to this study.
Through laboratory experiments, Werner’s team observed that when cancer cell mitochondria entered fibroblasts, these recipient cells experienced a “hyper boost” in growth. The mitochondria-rich fibroblasts began to proliferate more rapidly and activated genes associated with cancer. This reprogramming was significant enough to influence tumor formation when these modified fibroblasts were injected alongside cancer cells into mouse models.
The research identified a critical protein, MIRO2, which plays a key role in the mitochondrial transfer process. MIRO2 facilitates the transportation of mitochondria to the edges of cancer cells where these nanotubes form. The absence of MIRO2 hindered the cancer cells’ ability to transfer mitochondria, highlighting its importance in this cellular manipulation.
The implications of this work extend beyond fibroblasts. According to Yosuke Togashi, a molecular biologist at Okayama University, cancer cells can also deliver mitochondria to immune cells, which may suppress their ability to combat cancer effectively. Togashi’s team suggested this earlier in 2023, emphasizing the versatile role of mitochondrial transfer in the tumor microenvironment.
While the field of mitochondrial transfer is still emerging, it is gaining momentum. Jiří Neužil from the Czech Academy of Sciences remarked on the rapid growth of research in this area, expressing confidence that these interactions are indeed occurring. He noted the plethora of questions raised by Werner’s findings, including the driving forces and mechanisms behind this transfer.
The study exemplifies the value of pursuing unexpected findings in scientific research. Werner’s team initially set out to investigate cellular communication but stumbled upon a complex interplay that could redefine our understanding of cancer biology. The identification of mitochondrial dynamics in cancer opens new avenues for potential therapeutic targets, with MIRO2 being highlighted as a particularly promising candidate for future investigations.
As researchers delve deeper into the implications of mitochondrial transfer, the potential to develop innovative cancer therapies may transform how the disease is approached. Understanding these cellular interactions could lead to more effective strategies in combating the pervasive challenge of cancer.