A recent study from the University of Pennsylvania has unveiled a significant finding regarding the influence of parental DNA on telomere length during early embryonic development. The research indicates that not only do mothers and fathers pass on their genetic material to their offspring, but they also actively reshape telomere length, impacting aging and disease susceptibility, including conditions like cancer.
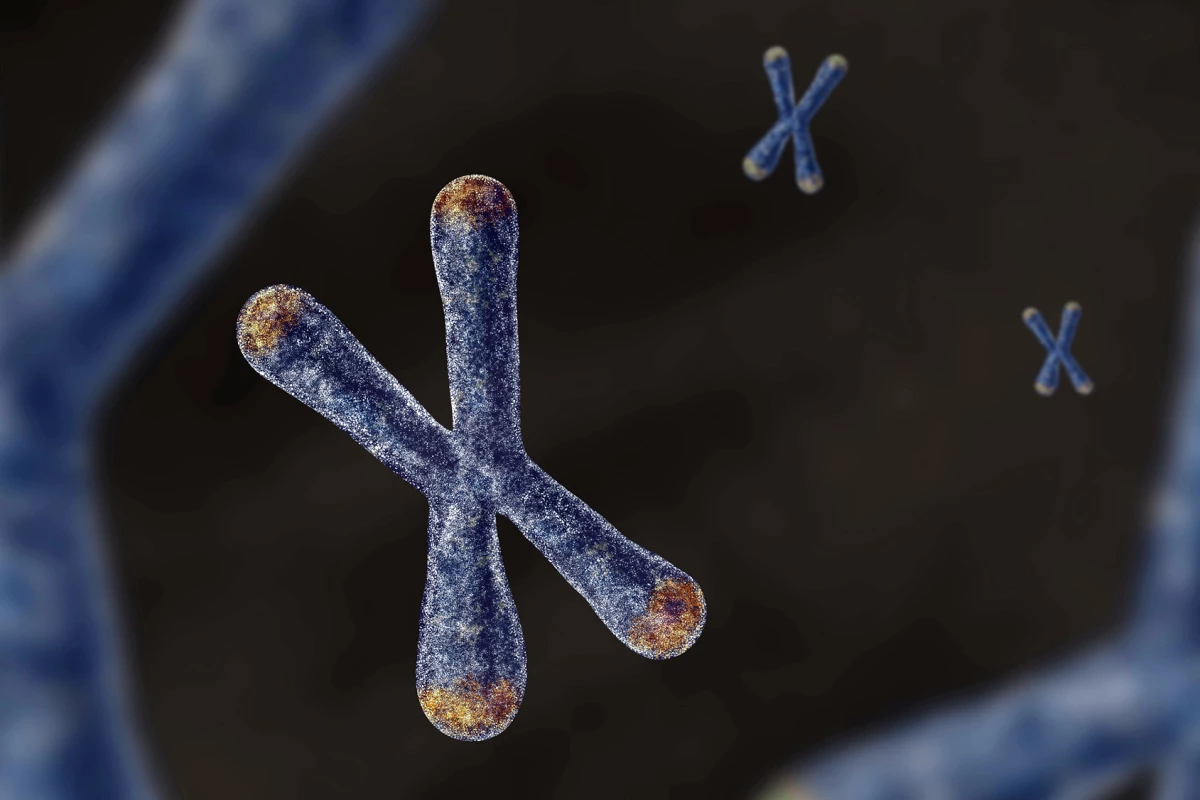
Telomeres, the protective caps at the ends of chromosomes, play a crucial role in safeguarding genetic information. However, each time a cell divides, these telomeres shorten. When they become too short, a process known as cellular senescence occurs, which has been linked to various diseases, particularly cancer. Traditionally, scientists viewed telomere length as a trait influenced by multiple genes or inherited directly from parental sperm and egg cells. This study introduces the concept of a “parent-of-origin effect,” which suggests that telomere length is actively modified during the initial stages of life.
Michael Lampson, PhD, a professor of biology at UPenn and a co-corresponding author of the study, stated, “We wanted to ask how telomeres are really inherited. Is it just the telomere DNA sequence you inherit from your parents, or is it determined by the genes that regulate telomeres? What we found doesn’t fit neatly into either box.”
To explore this concept, researchers conducted controlled experiments on mice, crossing strains with markedly different telomere lengths. They observed the telomere length in embryos from the two-cell stage onward, utilizing a DNA imaging technique known as fluorescent in situ hybridization (FISH). This method illuminates telomeres, allowing for precise measurement under a microscope.
Remarkably, findings revealed that the telomere length of the offspring did not simply average the lengths of the parents. In cases where paternal telomeres were long and maternal telomeres were short, embryos exhibited a rapid elongation of telomeres. Conversely, when the maternal telomeres were long and paternal telomeres were short, the opposite occurred. If both parents possessed similar telomere lengths, the offspring’s telomeres remained unchanged. Notably, these differences were detected as early as the two-cell stage, prior to the activation of the embryo’s own genes.
The study also identified molecular indicators of a mechanism known as alternative lengthening of telomeres (ALT), which extends telomeres without the typical enzyme telomerase. While most cells cease telomerase production after early development, many cancer cells can reactivate it, allowing for unlimited division—a key feature of tumor growth. ALT, on the other hand, is used by a smaller subset of cancers to achieve similar outcomes.
Mia Levine, PhD, an associate professor of biology at UPenn and the study’s other corresponding author, noted, “This parent-of-origin effect is consistent with patterns we’ve seen in human studies. For example, children of older fathers tend to have longer telomeres than children of younger fathers.”
The researchers acknowledged that human studies are complicated by numerous confounding factors, such as diet and lifestyle. This complexity led them to utilize a controlled animal model to investigate these phenomena directly.
The implications of this research are significant. Understanding how telomere length is inherited could enhance risk prediction for age-related conditions. Furthermore, insights into controlling telomere length during early development may pave the way for innovative approaches in regenerative medicine and fertility treatments.
Looking ahead, the researchers aim to establish whether their findings extend to humans. “On the human side, we’re now taking advantage of long-read genome sequencing,” Levine explained. “That lets us look directly at telomeres in family trios—mom, dad, and child—to ask if the same parent-of-origin effects we saw in mice are detectable in humans.”
The findings of this study were published in the journal Current Biology, marking a pivotal step in understanding the intricate relationship between genetics, aging, and disease.