Scientists have revealed significant insights into how mitochondria affect T-cell proliferation and memory formation. Led by Navdeep Chandel, Ph.D., a prominent figure in the Division of Pulmonary and Critical Care, this research published in Nature Immunology highlights the critical role of mitochondrial metabolism in preventing T-cell exhaustion, particularly in the context of cancer and chronic infections.
The research team focused on the mitochondrial electron transport chain (ETC), a series of protein complexes essential for energy production within cells. Previous studies from Chandel’s laboratory indicated that mitochondrial ETC function is vital for the proliferation of CD8 + T-cells, which are instrumental in combating cancer and viral infections. However, the specific roles of the mitochondrial ETC in promoting these immune responses had not been fully understood until now.
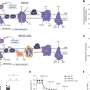
To explore these mechanisms, researchers engineered mice that lacked mitochondrial complex III, one of the four integral complexes in the ETC. This complex is responsible for transporting electrons and facilitating ATP generation, as well as producing reactive oxygen species (ROS) that serve as signaling molecules. Observations from these genetically modified mice revealed that the absence of complex III led to reduced cellular respiration and compromised ATP production, directly impacting the proliferation of CD8 + T-cells during viral infections.
Unexpectedly, the study showed that T-cells lacking mitochondrial complex III experienced rapid exhaustion following acute antigen stimulation, a phenomenon previously associated only with chronic antigen exposure. Furthermore, the impaired function of this mitochondrial complex hindered the formation of memory CD8 + T-cells, which are crucial for long-term immunity following initial infections.
“The immune system robustly responds to viral infections, clearing them effectively, but the presence of memory T-cells is what provides protection against future encounters with the same virus,” explained Chandel, who also holds positions at the Chan Zuckerberg Biohub and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
In a bid to further comprehend the mechanisms at play, the research team introduced a protein called alternative oxidase (AOX) derived from Ciona intestinalis into the CD8 + T-cells lacking mitochondrial complex III. This protein compensates for the loss of complex III by preventing ROS production. Results indicated that while AOX successfully mitigated T-cell exhaustion and restored cell metabolism and proliferation, it did not facilitate memory formation. This suggests that ROS generation plays a crucial role in the establishment of T-cell memory.
“We observed the expression of memory precursor markers in these cells, indicating that the issue is not merely an immediate death signal but rather a failure to develop a terminal memory cell capable of long-term persistence,” stated Elizabeth Steinert, Ph.D., the lead author of the study and a research assistant professor in Chandel’s division.
The findings emphasize the necessity of mitochondrial respiration for both T-cell proliferation and memory formation. This understanding could pave the way for innovative therapeutic strategies that target mitochondrial function in T-cells. Chandel remarked, “Mitochondrial metabolism is essential for preventing exhaustion, supporting proliferation, and generating memory. This positions mitochondria at the core of T-cell biology, suggesting that therapies aimed at rejuvenating them could be beneficial.”
The comprehensive study highlights the intricate relationship between mitochondrial function and immune cell behavior, offering promising avenues for future immunotherapy developments aimed at enhancing T-cell responses against cancer and chronic infections.
For more detailed information, refer to the publication: Elizabeth M. Steinert et al, “Mitochondrial respiration is necessary for CD8+ T cell proliferation and cell fate,” in Nature Immunology (March 2025). DOI: 10.1038/s41590-025-02202-x.