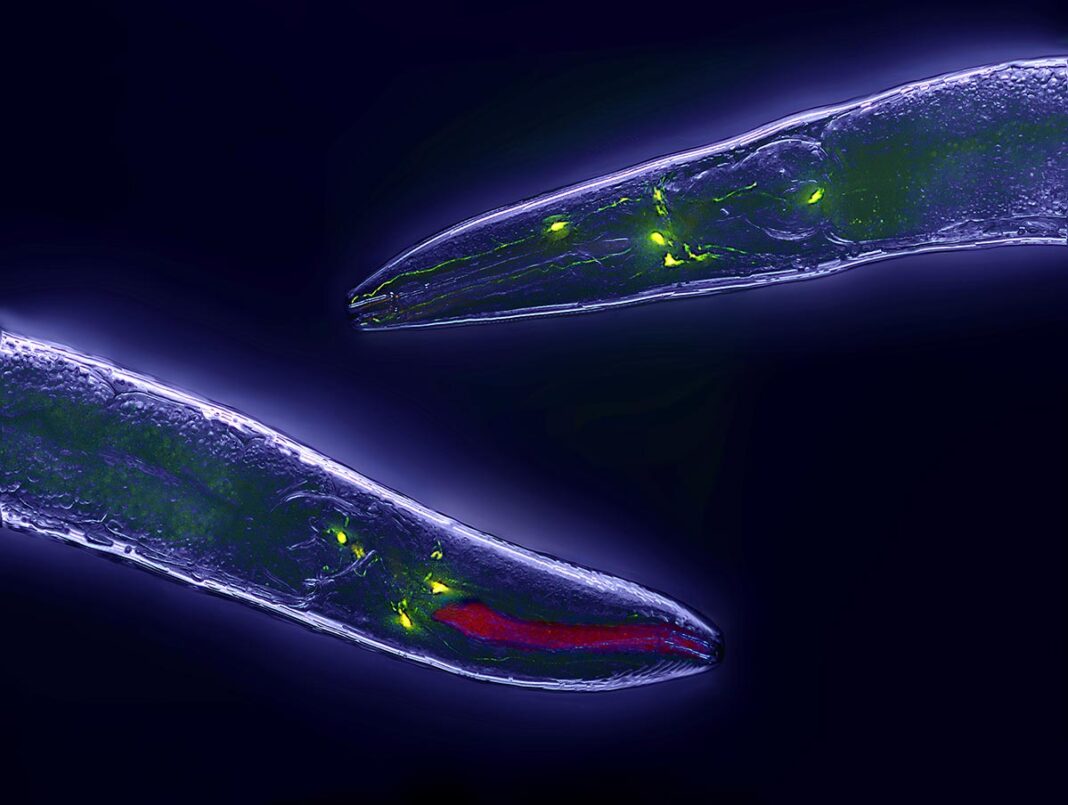
A groundbreaking study from scientists at the Howard Hughes Medical Institute (HHMI) has uncovered mechanisms responsible for the inheritance of longevity traits in the worm species C. elegans. Published in the journal Science, the research reveals how alterations in the lysosomes of parent worms can influence the lifespan of their offspring, even if those offspring do not possess the same genetic modifications.
The study is led by Dr. Meng Wang, a senior group leader at HHMI’s Janelia Research Campus. Previous findings from Wang’s laboratory indicated that overexpressing a specific enzyme in the lysosomes of C. elegans could extend their lifespan by as much as 60 percent. Intriguingly, the offspring of these genetically modified worms also exhibited increased longevity, suggesting a form of transgenerational inheritance.
Wang’s latest research builds on these earlier insights, exploring how longevity markers are passed down through generations. The team investigated the role of histones—proteins that package DNA— in transferring these longevity traits. Their findings indicate that changes in the lysosomes of C. elegans are communicated to reproductive cells via histone modifications. This process modifies the worm’s epigenome, allowing the lysosomal changes to be inherited without altering the fundamental DNA.
“You always think that your inheritance is in the nucleus, within the cell, but now we show that the histone can go from one place to another place,” Wang explained. She emphasized that if a histone carries any modifications, it transfers epigenetic information from one cell to another, providing a clearer understanding of transgenerational effects.
Through a combination of transcriptomics and imaging techniques, the researchers identified specific histone modifications that were notably elevated in long-lived worms compared to their shorter-lived counterparts. The study revealed that metabolic changes in lysosomes, particularly during fasting, initiate a cascade of cellular processes that enhance the production of a specific histone variant. This variant is then transported to reproductive cells through proteins, where it undergoes modifications that enable the longevity-related information to enter the germline.
The implications of this research extend beyond the lifespan of C. elegans. The mechanisms identified may also elucidate how various types of inherited information are transmitted from parents to offspring. For instance, the findings could offer insights into previously documented transgenerational effects, such as how a parent’s malnutrition impacts their offspring or how epigenetic adaptations to environmental stress are passed down.
As the scientific community continues to explore the complexities of inheritance, this research provides a significant step forward in understanding the role of epigenetic mechanisms in transgenerational health and longevity. The work of Wang and her team contributes to a growing body of evidence that challenges traditional views of genetic inheritance and opens new avenues for studying lifespan and health across generations.