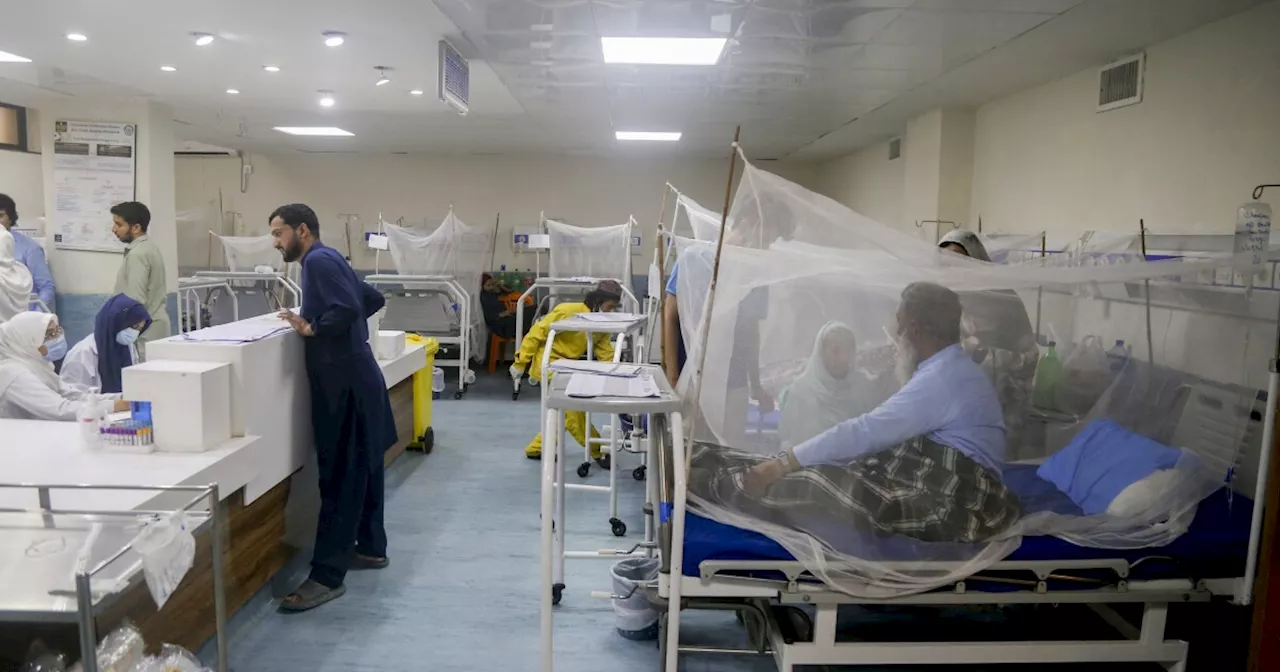
Researchers have made significant strides in the fight against malaria with the development of a new antimalarial drug, GanLum, which has shown promising results in clinical trials across Africa. This new medication, a combination of ganaplacide and lumefantrine, is positioned as a potential solution to the growing challenge of artemisinin-resistant malaria, a serious global health threat.
The fight against malaria, a disease responsible for hundreds of thousands of deaths each year, has seen remarkable advancements, particularly with the introduction of artemisinin-based treatments derived from the sweet wormwood plant. These treatments revolutionized malaria care in the early 2000s. However, the malaria parasite’s capacity to evolve and develop resistance to these drugs is raising alarms, echoing the history of previous antimalarials that eventually became ineffective.
The emergence of artemisinin resistance began in Southeast Asia in the late 2000s and has since spread to Africa, a region heavily impacted by the disease. This situation underscores the urgent need for new treatment options capable of countering the parasite’s defenses, emphasizing the importance of GanLum’s development.
GanLum is particularly noteworthy as it combines ganaplacide, a novel compound discovered by scientists at Novartis, with lumefantrine, an established antimalarial. Preliminary research suggests that GanLum disrupts the malaria parasite’s ability to thrive within human red blood cells. In laboratory tests, it effectively eliminates all known strains of the parasite, including those showing artemisinin resistance, which is a crucial factor in addressing this escalating concern.
Clinical Trials and Efficacy
Clinical trials involving over 16,000 patients across 12 African countries have indicated that GanLum is as effective, if not slightly superior, to current artemisinin-based treatments. While both treatment options exhibited similar side effects, such as nausea and diarrhea, the GanLum group reported a higher incidence of vomiting. This aspect will be a key consideration as the drug moves through the regulatory process, with expectations of it becoming available for patients in approximately a year and a half.
Despite GanLum’s potential, it is unlikely to completely replace artemisinin-based treatments in the immediate future, as these remain effective in many areas. However, with the growing threat of resistance, having alternative treatments like GanLum is essential. This could extend the effectiveness of both GanLum and existing artemisinin-based therapies, equipping healthcare professionals with a diverse set of tools to combat malaria.
The availability of GanLum could help prevent a resurgence of the high mortality rates associated with previous drug resistance crises. As healthcare systems prepare for the emergence of new resistance patterns, the development of this antimalarial serves as a critical safeguard, providing options that can prevent the devastating consequences of ineffective treatments.
In conclusion, the success of GanLum could transform malaria treatment protocols and save countless lives worldwide. The ongoing efforts of researchers, scientists, and health organizations remain fundamental in addressing malaria and minimizing the impact of treatment resistance. GanLum’s promising results shine a light on the continued fight against this deadly disease, underscoring the importance of innovation in medical research.