A significant international study has identified effective treatment options for chronic hives, known scientifically as urticaria, when standard antihistamines prove ineffective. Conducted by researchers at McMaster University in Canada, the findings provide essential guidance for both patients and healthcare professionals navigating this persistent condition.
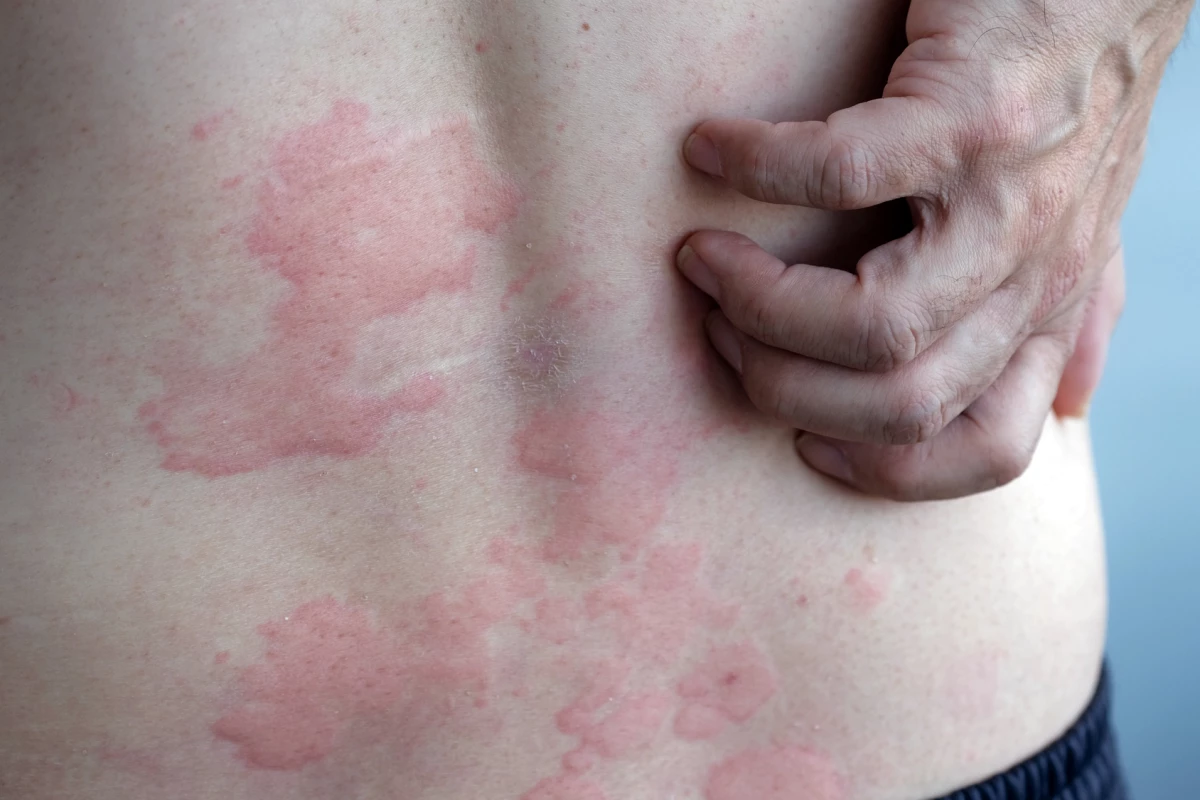
Chronic hives manifests through symptoms such as redness, swelling, and intense itching. When these symptoms persist for more than six weeks, they are classified as chronic. While antihistamines are generally the first-line treatment, they do not always deliver sufficient relief, leading to the exploration of systemic therapies that affect the entire body. This new research compiles data from 93 studies involving 11,398 participants, primarily adolescents and adults suffering from moderate to severe chronic urticaria symptoms.
The study, published in The Journal of Allergy and Clinical Immunology, represents the first comprehensive analysis of advanced treatment options for chronic urticaria. According to Derek Chu, MD, PhD, the corresponding author and an assistant professor in McMaster’s Department of Medicine, the results offer a clear, evidence-based framework from which patients and healthcare providers can choose appropriate treatments.
Researchers employed a systematic review and a Bayesian network meta-analysis (BNMA) to evaluate the safety and effectiveness of various medications and immunotherapies. They focused on outcomes that matter to patients, including the severity of itching and wheals, angioedema (swelling), quality of life, and any adverse events associated with treatment. Each study underwent rigorous evaluation for quality and risk of bias, with the certainty of evidence rated as high, moderate, low, or very low using the GRADE criteria.
Among the treatments ranked with high certainty of evidence, omalizumab (Xolair) administered at 300 mg every four weeks emerged as one of the most effective options. This injectable monoclonal antibody works by blocking the action of immunoglobulin E (IgE), a substance involved in allergic reactions and inflammation. Another promising treatment is remibrutinib, an oral medication that inhibits Bruton’s tyrosine kinase (BTK), targeting a pathway that leads to the release of histamine and other inflammatory mediators responsible for chronic hives.
While still not commercially available, remibrutinib has shown positive outcomes in Phase 3 clinical trials, indicating it could be a vital future treatment option. Additionally, dupilumab (Dupixent), another injectable immunotherapy, has shown potential in reducing itch and wheal severity by blocking interleukin-4 (IL-4) and interleukin-13 (IL-13), proteins that contribute to inflammation. However, its effectiveness in improving swelling and overall quality of life remains uncertain due to insufficient data.
The study also considered cyclosporine, an immunosuppressive medication that reduces inflammation. While it demonstrated effectiveness in decreasing itch and wheal severity, it was also associated with significant side effects, including kidney toxicity and elevated blood pressure.
Despite its comprehensive nature, the study has limitations. Most of the trials included were short-term, which poses challenges in assessing the long-term safety of some treatments. Additionally, older medications such as sulfasalazine and methotrexate received low evidence ratings mainly due to small or non-randomized studies. Children were underrepresented, with only one trial involving participants under the age of 12, limiting guidance for treating pediatric urticaria. Furthermore, the research did not include randomized controlled trials examining combinations of treatments, which are often used in practice.
Nonetheless, this extensive study provides a clearer hierarchy of treatment options beyond antihistamines, allowing for more personalized care. As patients and clinicians weigh their choices, practical considerations such as cost, convenience, and the patient’s comfort with injections will factor into decision-making.
The research was funded by the American Academy of Allergy, Asthma and Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI). The study marks a significant advancement in the understanding and treatment of chronic urticaria, offering hope to those searching for effective relief.