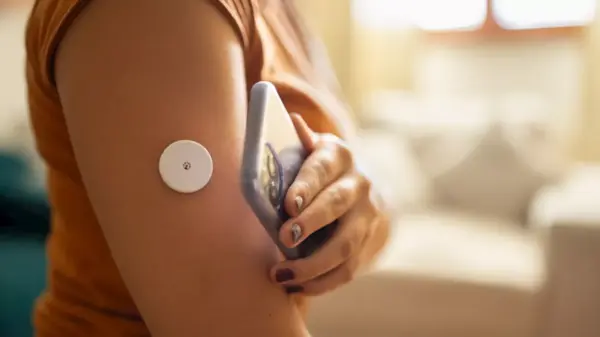
The U.S. Food and Drug Administration (FDA) has issued a serious alert regarding two glucose monitor sensors from Abbott Diabetes Care that have been linked to multiple fatalities. The agency reported that these sensors, the FreeStyle Libre 3 and FreeStyle Libre 3 Plus, are providing inaccurate low glucose readings, which could lead to critical health risks for users.
As of November 14, 2023, Abbott has documented 736 serious injuries and seven deaths associated with the malfunctioning devices. This alarming situation has prompted the FDA to classify the issue as a “potentially high-risk” concern. The malfunction stems from a manufacturing defect that Abbott has since acknowledged and corrected.
In a statement, the FDA noted that Abbott had previously informed customers about the affected sensors in a press release dated November 24, 2023. The company reported that over 3 million FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors are impacted. These devices are essential for diabetes management, especially for individuals aged four and older, by helping them monitor their glucose levels.
Abbott explained that the incorrect low readings could lead to dangerous treatment decisions, such as excessive carbohydrate intake or inappropriate insulin dosing. “If undetected, incorrect low glucose readings over an extended period may lead to serious health risks, including potential injury or death,” the company stated.
As a precaution, Abbott has advised customers to stop using the affected sensors immediately and to dispose of them safely. The specific models affected include:
– **FreeStyle Libre 3 Sensor**
– Model Numbers: 72081-01, 72080-01
– Unique Device Identifiers: 00357599818005, 00357599819002
– **FreeStyle Libre 3 Plus Sensor**
– Model Numbers: 78768-01, 78769-01
– Unique Device Identifiers: 00357599844011, 00357599843014
Customers can locate the serial number on their devices through the app or reader, or on the label on the bottom of the sensor or carton. A full list of affected lot numbers is available on Abbott’s website.
In response to the crisis, Abbott is providing free replacements for the affected sensors. Customers can check the status of their devices at www.FreeStyleCheck.com and request a replacement if necessary. The company has stated that it has reached out to affected customers to offer support and guidance on next steps.
The FDA is encouraging anyone who has experienced adverse reactions or issues with their sensors to contact Abbott Diabetes Care at 1-833-815-4273. Additionally, both healthcare professionals and consumers are urged to report any problems to the FDA’s MedWatch program, which is dedicated to monitoring safety information and adverse events.
This recall underscores the critical nature of accurate glucose monitoring for individuals managing diabetes. The FDA and Abbott are committed to ensuring the safety and efficacy of medical devices, and this alert serves as a reminder of the importance of vigilance in health management.