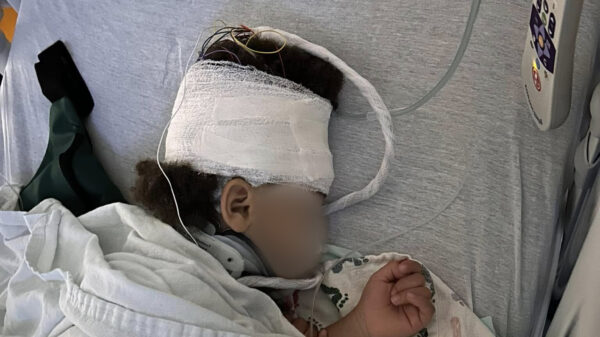
A federal judge has dismissed a significant case against Abbott Laboratories, relating to the safety of its formula for preterm infants. This decision may influence numerous similar lawsuits filed against the company. The case involved Keosha Diggs, a Maryland mother who claimed her son suffered from the life-threatening intestinal disease known as necrotizing enterocolitis (NEC) after being fed a specialized cow’s milk formula produced by Abbott.
Born at just 32 weeks of gestation, Diggs’ son required surgery to remove a portion of his intestine due to the illness. On March 1, 2024, U.S. District Judge Rebecca Pallmeyer granted Abbott’s motion for summary judgment, several weeks after indicating her decision at a pre-trial hearing. This case was poised to be the first federal trial in Chicago addressing whether Abbott’s cow’s milk-based formula contributes to NEC in preterm infants.
Hundreds of lawsuits concerning specialized formulas for preterm infants have been consolidated in the federal court in Chicago. Abbott has disclosed that it is currently facing over 1,400 lawsuits on this matter across the United States. The case involving Diggs was classified as a bellwether, meaning its outcome could guide the direction of similar cases in the federal court.
In a statement following the ruling, attorney James Hurst, representing Abbott, expressed appreciation for the court’s thorough examination of the case. Hurst stated, “Abbott has always believed these lawsuits are contrary to both the science and the law. We remain confident that Abbott will continue to prevail when the science is properly evaluated.”
Diggs had alleged that Abbott failed to provide adequate warnings to parents and healthcare providers regarding the risks associated with NEC when using their specialized products. In her opinion issued on March 1, Judge Pallmeyer noted that the testimony of an expert witness, who claimed that cow’s milk-based formulas could contribute to NEC in preterm infants, was not admissible in court. The judge agreed with Abbott that this expert’s testimony was irrelevant because it applied to infants who were born earlier and weighed less than Diggs’ son.
Without this expert testimony, the judge concluded that Diggs could not demonstrate a direct link between the formula and her son’s illness. She also highlighted several deficiencies in Diggs’ case, which mirrored issues observed in another bellwether case that she had dismissed earlier this year.
Legal analysts from Wells Fargo commented that the ruling indicates a challenging path ahead for claimants. They noted that Abbott and Mead Johnson, the formula manufacturer, possess strong defenses against the allegations. For years, both companies have faced lawsuits from parents who assert that their premature infants became ill after consuming these specialized formulas. These parents contend that the manufacturers should have issued stronger warnings regarding the potential dangers of cow’s milk-based products for preterm infants.
Despite the ongoing legal disputes, the scientific community remains divided on the issue. While research indicates a correlation between formula feeding and increased rates of NEC among preterm infants, it does not establish causation. In a statement released last year, three federal agencies—the U.S. Food and Drug Administration, the Centers for Disease Control and Prevention, and the National Institutes of Health—asserted, “There is no conclusive evidence that preterm infant formula causes NEC.”
Medical professionals caution that these specialized formulas are vital nutritional sources for many infants, particularly when breast milk is unavailable. They worry that continued litigation could lead Abbott to withdraw these formulas from the market entirely. Though these products represent a small fraction of Abbott’s overall sales, their significance for neonatal care is profound.
In addition to the pending federal cases, three similar cases have already been adjudicated in state courts, yielding inconsistent results. One case resulted in a substantial verdict of $60 million against Mead Johnson, while another concluded with a $495 million judgment against Abbott Laboratories. Abbott is currently appealing this decision. In a separate case, a jury initially ruled that Abbott and Mead Johnson were not liable for a child developing NEC after consuming their products. However, a St. Louis judge later granted a new trial due to reported “errors and misconduct” during the original proceedings.
As the legal battles continue, the implications of these court decisions reach beyond Abbott and Mead Johnson, affecting the broader conversation about infant nutrition and the responsibilities of formula manufacturers.